Sample Solution
bchct-131-solved-assignment-2024-ss-10cd7422-4b11-4531-9171-a6445826d855
BCHCT-131 Solved Assignment 2024
PART-(A)
- Using a suitable diagram explain the spectral transitions between different energy levels of hydrogen atom. Also name these series of lines and give the region of electromagnetic radiation in which they appear.
Answer :
The spectral transitions in a hydrogen atom can be explained with the help of a diagram showing the various energy levels and the transitions between them. In a hydrogen atom, the electron can occupy various energy levels, which are quantized. The energy levels are often denoted by the principal quantum number n n n=1,2,3,dots n = 1, 2, 3, \ldots n=1 n = 1
When an electron transitions from a higher energy level to a lower energy level, it emits a photon. The energy of this photon corresponds to the difference in energy between the two levels. These emissions form the spectral lines, which are grouped into series depending on the lower energy level to which the electron transitions.
The main series in the hydrogen spectrum are:
- Lyman Series: Transitions from higher energy levels
n >= 2 n \geq 2 n=1 n = 1 - Balmer Series: Transitions from higher energy levels
n >= 3 n \geq 3 n=2 n = 2 - Paschen Series: Transitions from higher energy levels
n >= 4 n \geq 4 n=3 n = 3 - Brackett Series: Transitions from higher energy levels
n >= 5 n \geq 5 n=4 n = 4 - Pfund Series: Transitions from higher energy levels
n >= 6 n \geq 6 n=5 n = 5
Let’s create a diagram to illustrate these transitions and energy levels in a hydrogen atom. This diagram will include energy levels represented by horizontal lines, with transitions between them shown as arrows. Each series of lines will be labeled, along with the region of the electromagnetic spectrum where they appear.
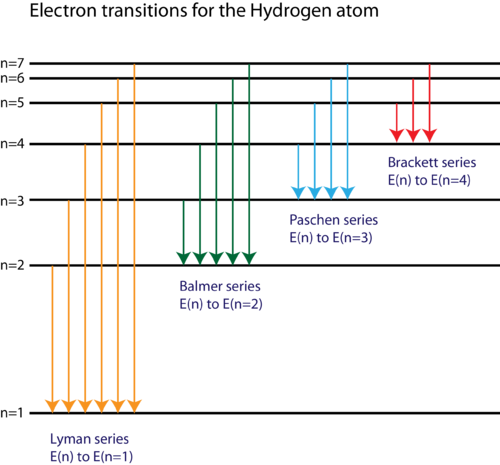
The illustration above shows the energy levels in a hydrogen atom. Each horizontal line represents a different energy level, labeled with the principal quantum number n n
- Lyman Series: This series represents transitions from higher energy levels to the
n=1 n = 1 - Balmer Series: These transitions occur from higher levels to the
n=2 n = 2 - Paschen Series: Transitions to the
n=3 n = 3 - Brackett Series: These are transitions to the
n=4 n = 4 - Pfund Series: This series includes transitions to the
n=5 n = 5
Each series corresponds to a distinct region in the electromagnetic spectrum, as indicated in the diagram. These spectral lines are fundamental in understanding atomic structure and the quantization of energy levels.
- What was the purpose of Davisson and Germer experiment? Explains and analyse its results.
Answer :
The Davisson and Germer experiment, conducted by American physicists Clinton Davisson and Lester Germer in 1927, was a pivotal experiment in quantum mechanics. Initially, its purpose was not to test any specific quantum hypothesis, but rather, it was an accidental discovery that came out of their study of the physical nature of electrons.
Original Purpose:
Davisson and Germer were initially investigating the surface of nickel crystals by bombarding them with electrons and observing how these electrons scattered off the surface. Their goal was to study the detailed structure of the nickel crystal by analyzing the patterns formed by scattered electrons.
Accidental Discovery and Quantum Mechanics:
However, the experiment ended up providing the first experimental confirmation of wave-particle duality, a fundamental concept in quantum mechanics. This duality, suggested by French physicist Louis de Broglie, proposed that particles such as electrons could exhibit both particle-like and wave-like properties.
Experiment Details:
In the experiment, Davisson and Germer directed a beam of electrons at a nickel crystal. According to classical physics, the electrons should scatter in a predictable pattern based on their particle-like interactions with the nickel atoms. However, the results were quite different.
Results and Analysis:
- Wave-Like Behavior: Instead of a random scattering pattern, Davisson and Germer observed a series of concentrated spots. These spots formed a pattern that resembled the diffraction patterns produced by waves, not particles. When the wavelength of these electron waves was calculated, it matched the theoretical prediction made by de Broglie for the wavelength of an electron.
- Confirmation of de Broglie’s Hypothesis: This experiment provided the first solid evidence of de Broglie’s hypothesis of matter waves, suggesting that matter can exhibit wave-like properties, a fundamental concept in quantum mechanics.
- Impact on Physics: The Davisson-Germer experiment’s findings were groundbreaking. They played a significant role in the acceptance of wave-particle duality theory and the broader development of quantum mechanics. This theory fundamentally altered our understanding of how particles at the atomic and subatomic level behave.
- Brillouin Zones Analysis: Later analyses related the experiment’s results to the concept of Brillouin zones in solid state physics, further illustrating the wave nature of electrons and their interactions in crystalline structures.
In summary, the Davisson-Germer experiment, which began as a study of electron scattering, accidentally became one of the foundational experiments in quantum mechanics, providing concrete evidence for the wave-particle duality of matter. This experiment not only confirmed de Broglie’s hypothesis but also paved the way for the development of new theories and models in quantum physics.
Verified Answer
Rated 5 out of 5