₹101.00
Share with your Friends
IGNOU BCHCT-131 Assignment Question Paper 2023
Course Code: BCHCT-131
Assignment Code: BCHCT-131/TMA/2023
Maximum Marks: 100
Note: Attempt all questions. The marks for each question are indicated against it.
\section{PART-(A)}
1. Derive the following expression for the energy of electron in the \(n^{\text {th }}\) orbit.
\(E_{n}=\frac{-Z^{2} e^{2} m}{8 \varepsilon_{o} h^{2} n^{2}}\)
2. What is a black body? Discuss the important features of black body radiation giving a suitable diagram.
3. Derive the time independent Schrodinger equation for a particle.
4. Draw the shapes of \(5 d\) orbitals. Also indicate the signs of wave functions and nodal planes in the diagram.
5. Write the electronic configurations for the following elements.
(i) \(\mathrm{Cr}\)
(ii) \(\mathrm{Mo}\)
(iii) \(\mathrm{Ag}\)
Also explain your answer.
6. (a) Write down Born-Haber cycle for \(\mathrm{CaCl}_{2}\) formation.
(b) Discuss the factors which affect the solubilities of ionic solids in water.
7. Write the assumptions of calculating the formal charge in a molecule. Calculate the formal charge in \(\mathrm{CH}_{3}-\mathrm{C}=\mathrm{O}-\mathrm{CH}_{3}\).
8. Draw the resonance structures of \(\mathrm{HCl}\). Out of them which one has little importance as a resonance structure and why?
9. (a) Calculate the percentage ionic character in \(\mathrm{HBr}\) gas.
Use following data:
The dipole moment of \(\mathrm{HBr}=2.635 \times 10^{-30} \mathrm{C} \mathrm{m}\)
Bond length of \(\mathrm{HBr}=141 \mathrm{pm}\).
(b) An anion will be more easily polarized, if it is large and highly negatively charged. Explain using suitable examples.
10. Draw and explain the molecular orbitals formed by the linear combination of following atomic orbitals
(i) \(p_{\mathrm{x}}\) and \(p_{\mathrm{x}}\) orbitals
(ii) \(p_{\mathrm{y}}\) and \(p_{\mathrm{y}}\) orbitals 11. Explain the following giving suitable examples:
(i) Position isomerism
(ii) Functional group isomerism
(iii) Chiral centre
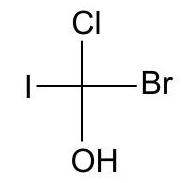
12. (a) Write two more Fischer projection formulas for the following compound:
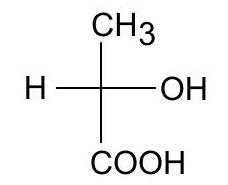
(b) Write the enantiomer of the following compound and assign their configurations as \(R\) or \(S\)
13. Draw the possible conformations of cyclohexane and explain their relative stability.
14. (a) Arrange the following compounds in the increasing order of their acidities and give reason in support of your answer.
3-chlorobutanoic acid, butanoic acid, 4-chlorobutanoic acid and 2-chlorobutanoic.
(b) Write acid the resonance structures of propanoate ion.
15. What is \(\mathrm{p} K_{\mathrm{a}}\) ? How does it help in explaining the basicity of different nucleophiles? Write a nucleophilic reaction indicating the nucleophile and its conjugate acid.
16. (a) How would you prepare cyclopentane starting from barium adipate? Write the reactions involved.
(b) Explain the term pyrolysis and cracking giving suitable examples.
17. (a) Explain Saytzeff rule giving a suitable example.
(b) Explain the mechanism of Birch reduction.
18. (a) How would you prepare 2-propanol from propene? Write the steps involved in the conversion.
(b) Write the products of ozonolysis of 2-methyl-2-butene.
19. Give the mechanism of hydration of ethyne and the product form.
20. Giving suitable diagrams, explain the structure of benzene.
BCHCT-131 Sample Solution 2023
Frequently Asked Questions (FAQs)
You can access the Complete Solution through our app, which can be downloaded using this link:
Simply click “Install” to download and install the app, and then follow the instructions to purchase the required assignment solution. Currently, the app is only available for Android devices. We are working on making the app available for iOS in the future, but it is not currently available for iOS devices.
Yes, It is Complete Solution, a comprehensive solution to the assignments for IGNOU. Valid from January 1, 2023 to December 31, 2023.
Yes, the Complete Solution is aligned with the IGNOU requirements and has been solved accordingly.
Yes, the Complete Solution is guaranteed to be error-free.The solutions are thoroughly researched and verified by subject matter experts to ensure their accuracy.
As of now, you have access to the Complete Solution for a period of 6 months after the date of purchase, which is sufficient to complete the assignment. However, we can extend the access period upon request. You can access the solution anytime through our app.
The app provides complete solutions for all assignment questions. If you still need help, you can contact the support team for assistance at Whatsapp +91-9958288900
No, access to the educational materials is limited to one device only, where you have first logged in. Logging in on multiple devices is not allowed and may result in the revocation of access to the educational materials.
Payments can be made through various secure online payment methods available in the app.Your payment information is protected with industry-standard security measures to ensure its confidentiality and safety. You will receive a receipt for your payment through email or within the app, depending on your preference.
The instructions for formatting your assignments are detailed in the Assignment Booklet, which includes details on paper size, margins, precision, and submission requirements. It is important to strictly follow these instructions to facilitate evaluation and avoid delays.
Terms and Conditions
- The educational materials provided in the app are the sole property of the app owner and are protected by copyright laws.
- Reproduction, distribution, or sale of the educational materials without prior written consent from the app owner is strictly prohibited and may result in legal consequences.
- Any attempt to modify, alter, or use the educational materials for commercial purposes is strictly prohibited.
- The app owner reserves the right to revoke access to the educational materials at any time without notice for any violation of these terms and conditions.
- The app owner is not responsible for any damages or losses resulting from the use of the educational materials.
- The app owner reserves the right to modify these terms and conditions at any time without notice.
- By accessing and using the app, you agree to abide by these terms and conditions.
- Access to the educational materials is limited to one device only. Logging in to the app on multiple devices is not allowed and may result in the revocation of access to the educational materials.
Our educational materials are solely available on our website and application only. Users and students can report the dealing or selling of the copied version of our educational materials by any third party at our email address (abstract4math@gmail.com) or mobile no. (+91-9958288900).
In return, such users/students can expect free our educational materials/assignments and other benefits as a bonafide gesture which will be completely dependent upon our discretion.
Related products
- IGNOU Assignment Solution
IGNOU MEG-19 Solved Assignment 2022-2023 | MEG | THE AUSTRALIAN NOVEL
₹101.00 Go to the App - IGNOU Assignment Solution
IGNOU MEG-15 Solved Assignment 2022-2023 | MEG | Comparative Literature: Theory and Practice
₹101.00 Go to the App - IGNOU Assignment Solution
IGNOU MEG-09 Solved Assignment 2022-2023 | MEG | Australian Literature
₹101.00 Go to the App